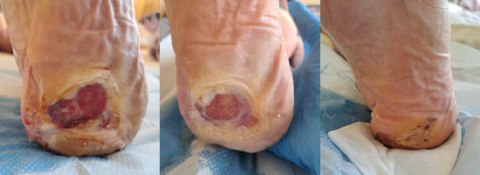
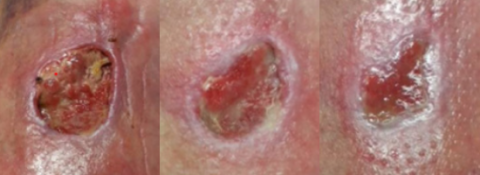
A problem that spreads far beyond the wound
Living with a non-healing wound can deeply affect patients' quality of life, from pain and sleep deprivation to social anxiety and isolation. Data highlight why the broader impact of non-healing wounds is such an enormous challenge. Flip the cards to reveal the problem.
Targeting progress
Clinicians in the post-acute space already dedicate a substantial amount of time to managing chronic wounds,4 so wounds that fail to progress add to that workload. Wounds that remain in a prolonged inflammatory phase beyond 4-6 weeks should be addressed promptly to improve the chances of recovery. The longer you wait, the more challenging it becomes to heal.5
When incorporated into a clinical pathway, evidence shows why PICO sNPWT can be an incredibly powerful tool to help activate healing in stalled wounds.1-3
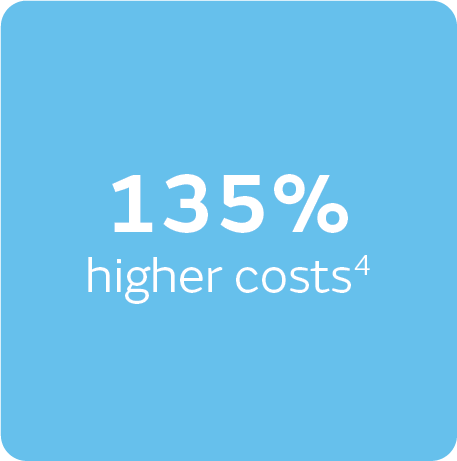
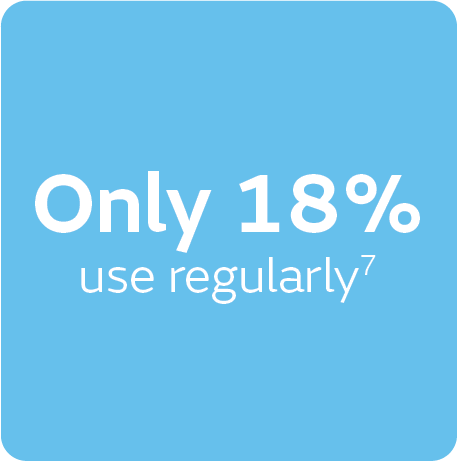
It’s estimated that community clinicians spend 43% of their time managing chronic wounds,4 and when up to 4.3 dressing changes per week (shown in DFUs)4 are combined with 135% higher treatment costs7 for non-healing wounds (vs healed wounds), it quickly adds up. Conversely, UK data showed only 18% of clinicians regularly use NPWT6 – a potentially powerful solution.
Why are clinicians using PICO sNPWT?
When building an effective treatment pathway for non-healing wounds, experts like Rosemary Hill need the option of advanced technologies at their disposal. Listen to Rosemary explain how and why she uses PICO sNPWT’s active therapy to help put wounds on a healing trajectory.
Kick-start healing with PICO sNPWT
If left unaddressed, the healing trajectory of non-healing wounds is unlikely to change…but clinical data has shown how the effective use of PICO sNPWT active therapy can help kick-start healing.
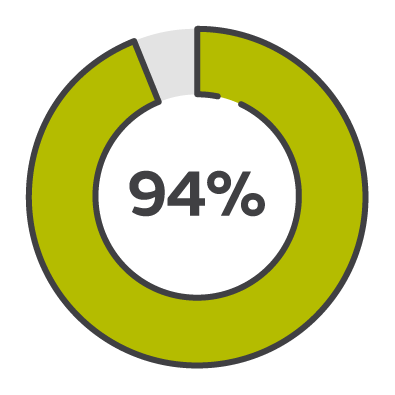
94% success rate
PICO sNPWT is more successful than standard dressings and tNPWT in putting wounds on a healing trajectory, turning around healing in just 2 weeks.3
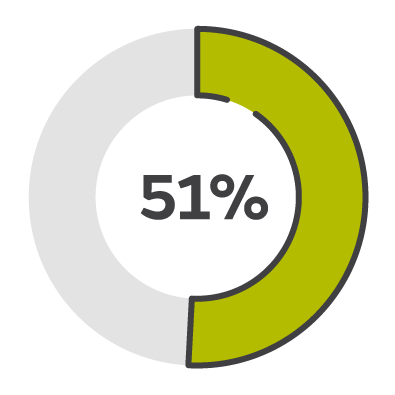
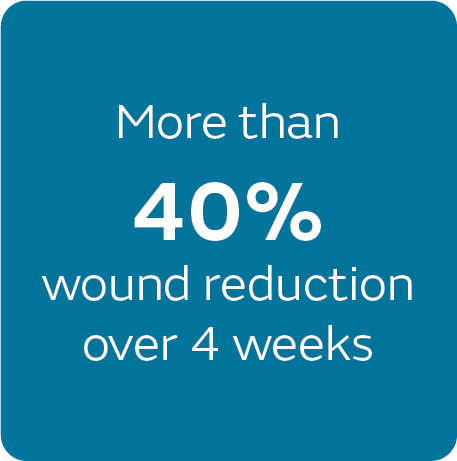
51% wound closure
Compared to traditional NPWT, PICO sNPWT achieved a greater reduction in wound area and depth, and a 51% relative increase in DFU and VLU wound closure.2
Discover PICO sNPWT
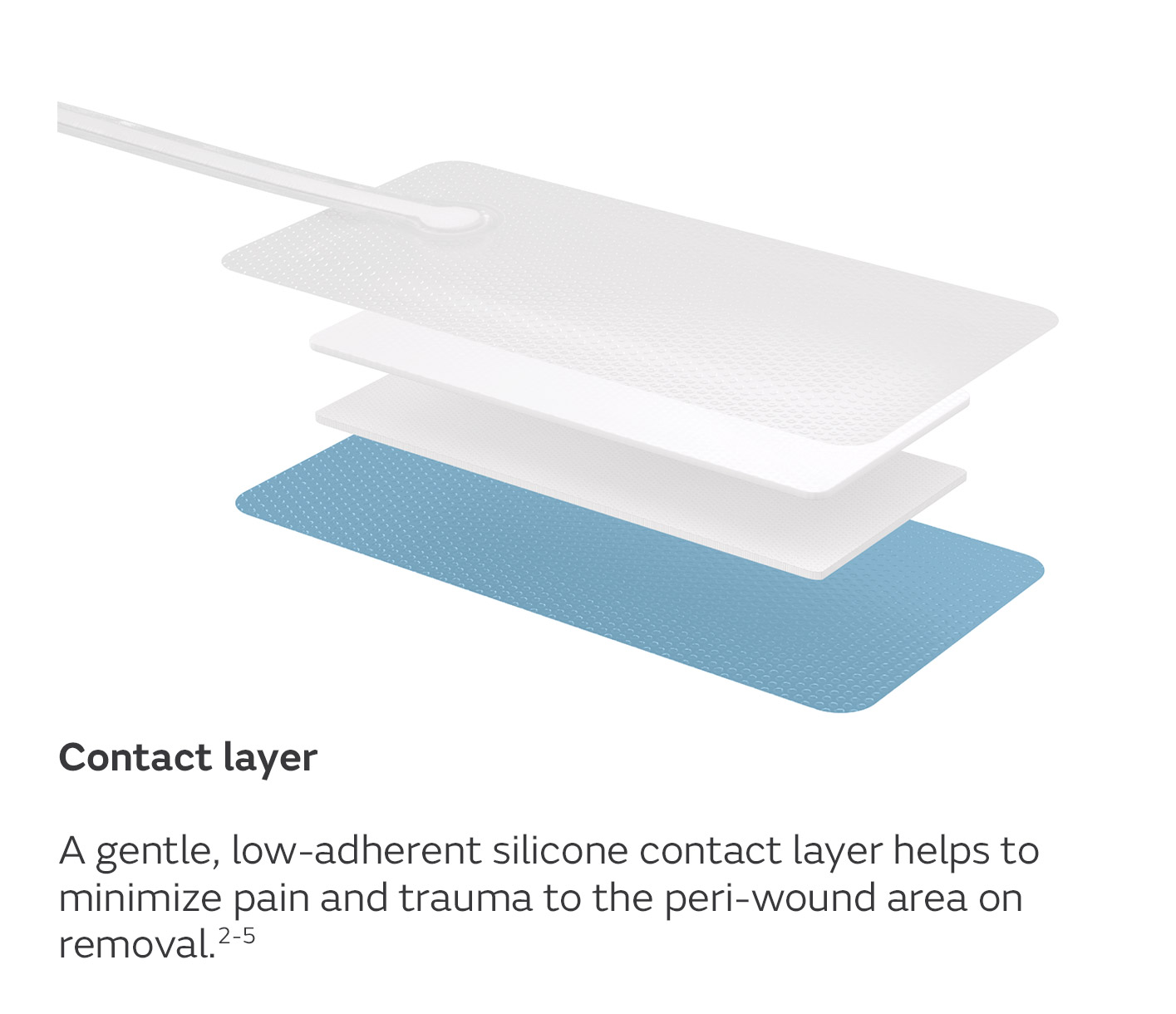
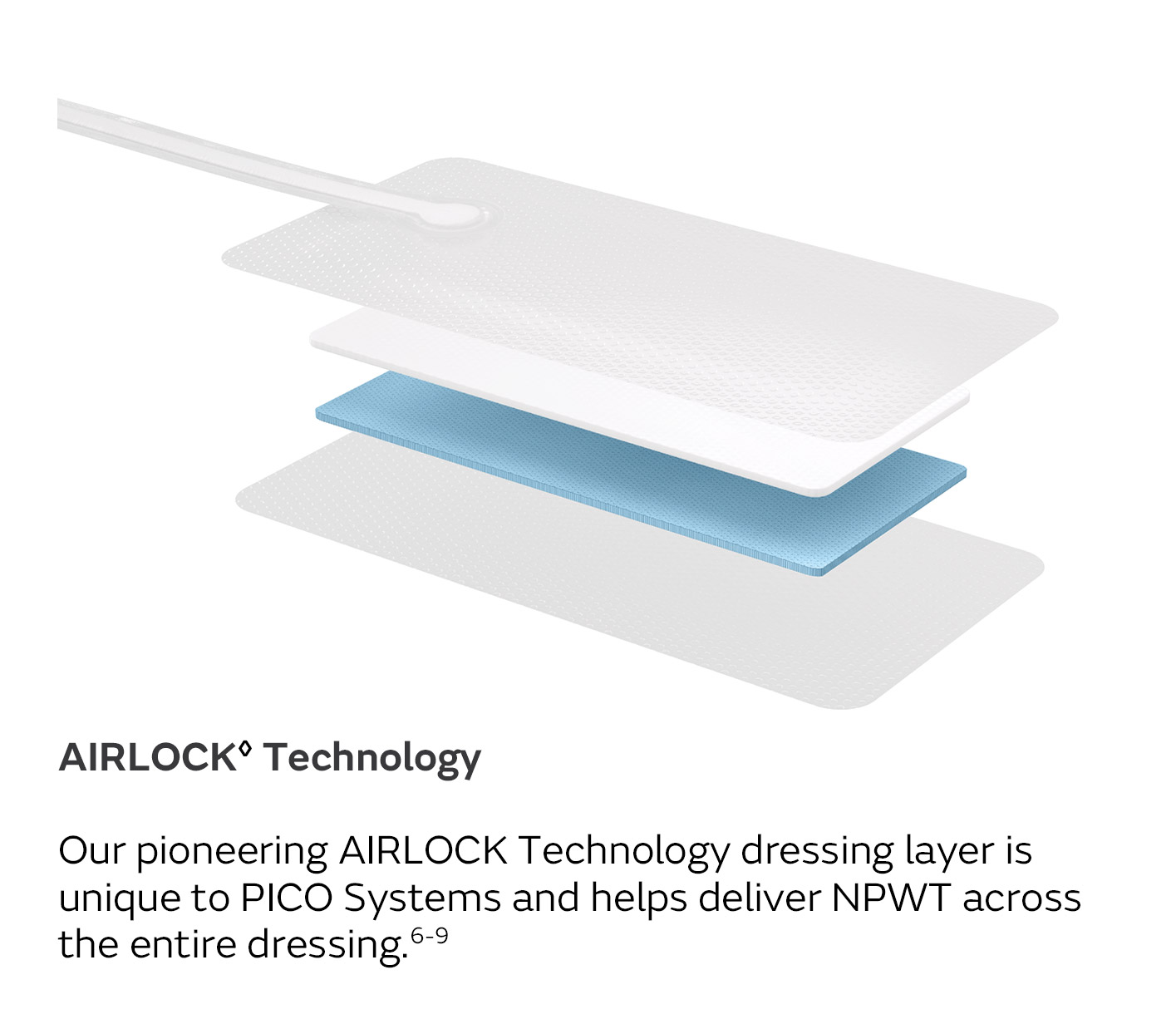
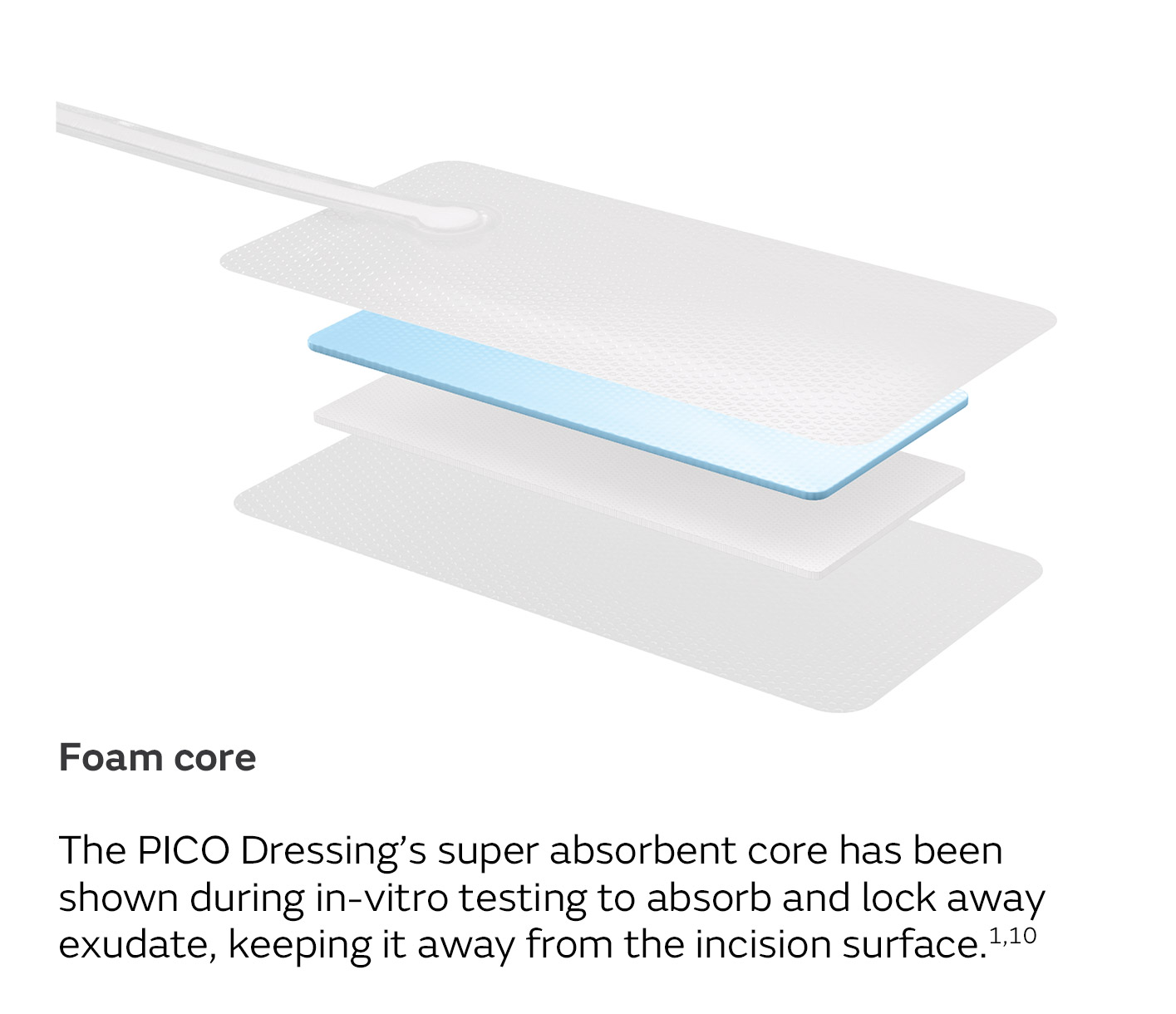
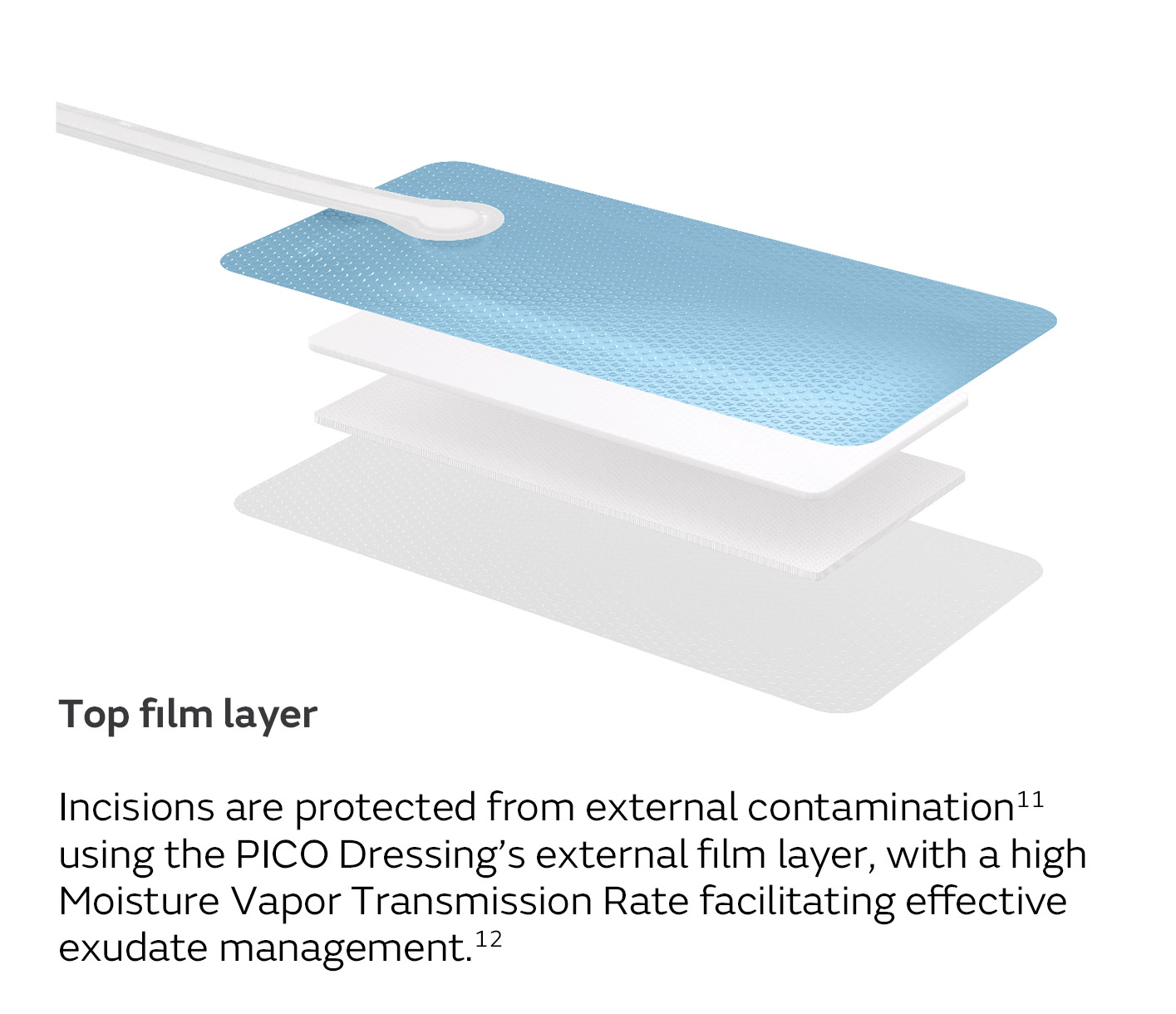
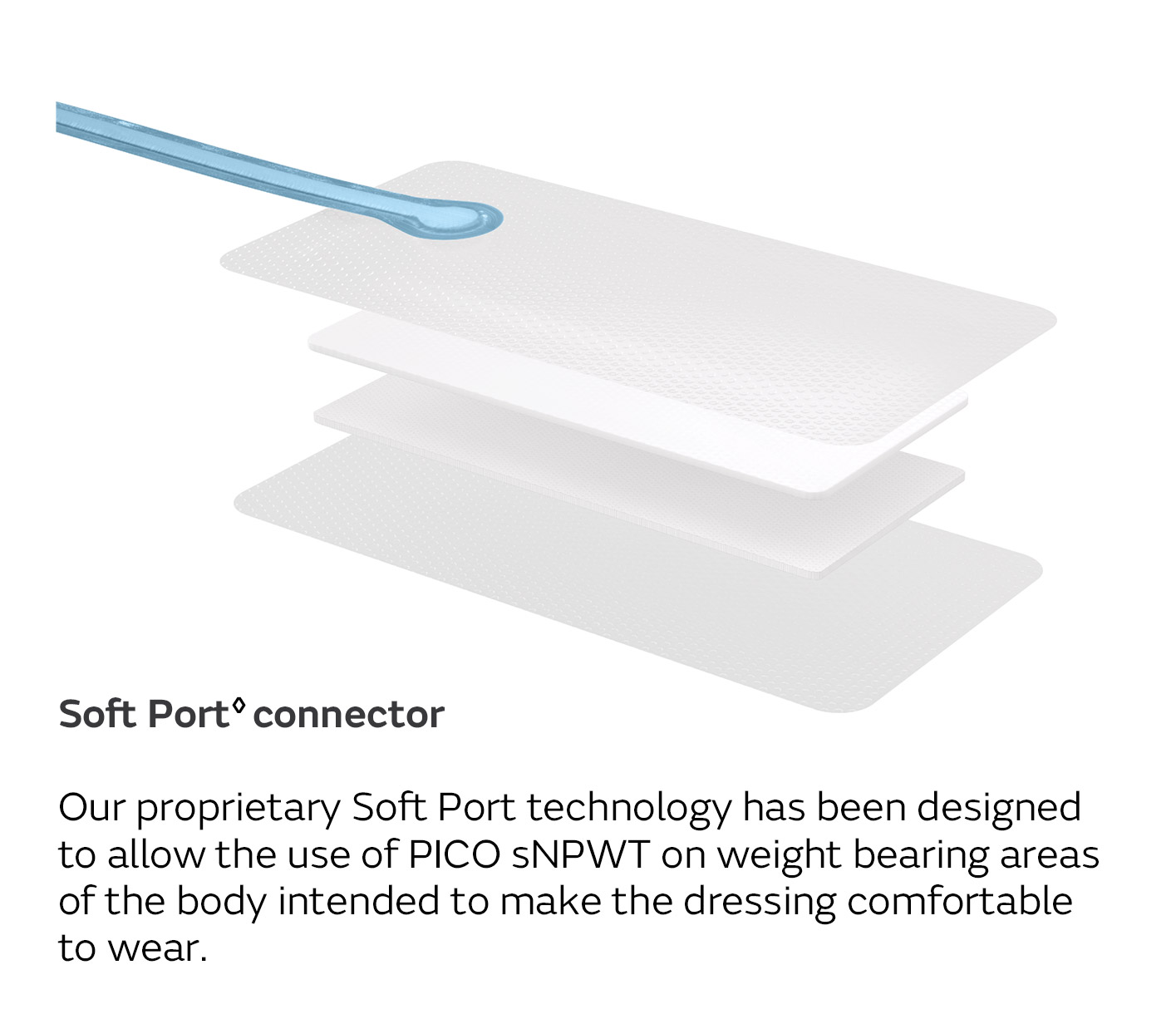
A unique four-layer, absorbent wound dressing10 connects to a lightweight, portable pump with visual alerts for air leaks, low battery and dressing changes.
- Developed to be pocket-sized and convenient, without the need for an exudate canister, so patients have a greater sense of freedom while undergoing NPWT.
- Suitable for safe and effective use in outpatient settings as it is easy to apply and remove13-15
- Can be used with fillers on deep wounds and is compatible with compression therapy5,16-19
Evidence shows that using PICO sNPWT as an active treatment, with its multiple mechanisms of action, can help improve healing rates in non-healing wounds, thereby helping reduce associated nursing resources and costs (compared to standard dressings).1-3
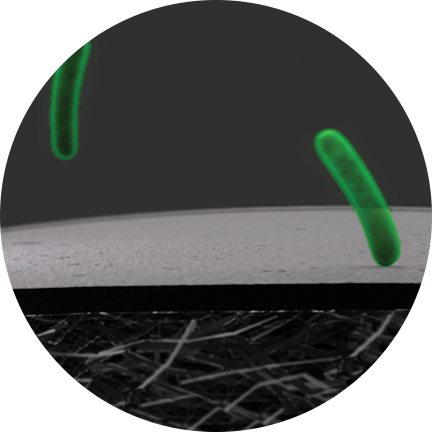
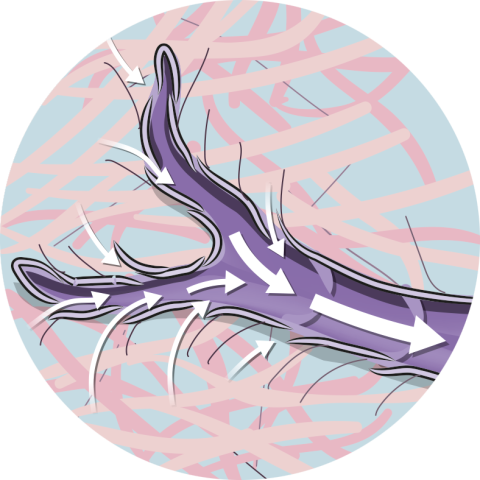
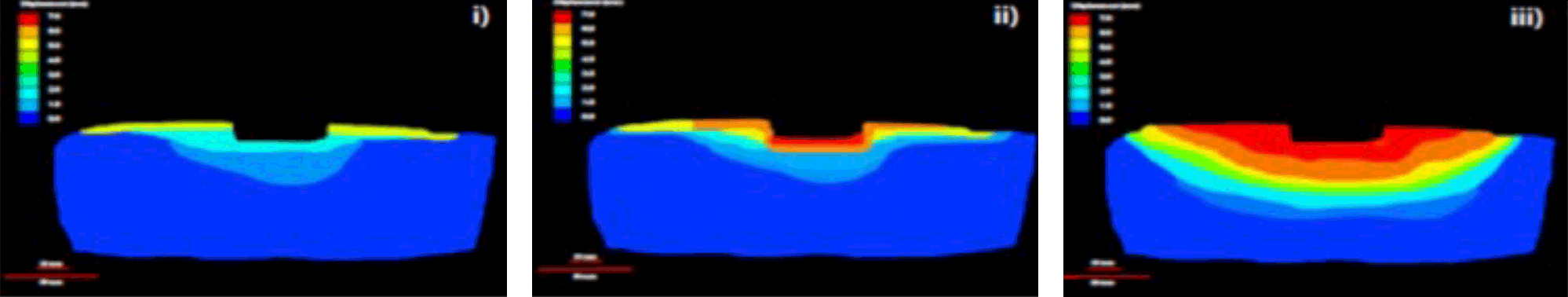
The PICO System's mode of action
Wider zone of compression
AIRLOCK Technology provides consistent delivery of NPWT (nominal 80mmHg) over a wide zone, beyond the wound itself, allowing for effective NPWT over the wound and periwound area.20
PICO sNPWT delivers compressive forces to tissues that span the entire dressing.20,42
The longer you wait, the harder it gets
Achieving successful outcomes depends on early and accurate assessment, identification of wound aetiology and consideration of local and systemic factors that may be contributing to non-healing.
Evidence has demonstrated that healing success rates become gradually less favourable if active therapy is implemented after 3 months.2 Aim to follow a clinical pathway that facilitates early intervention on suitable patients.
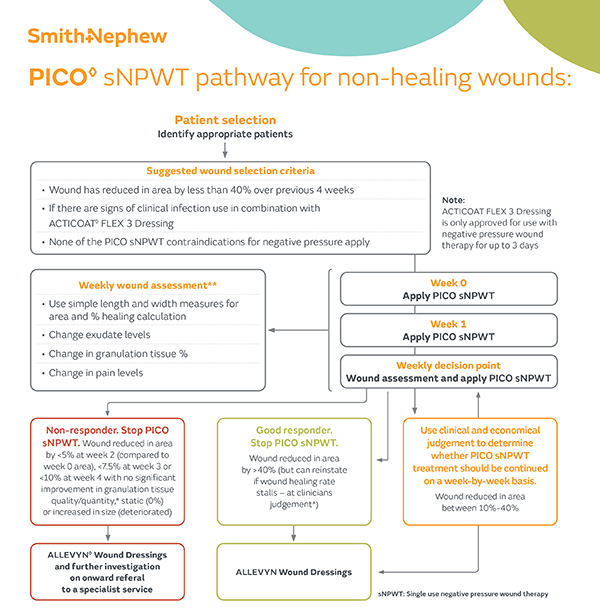
Implementing PICO sNPWT
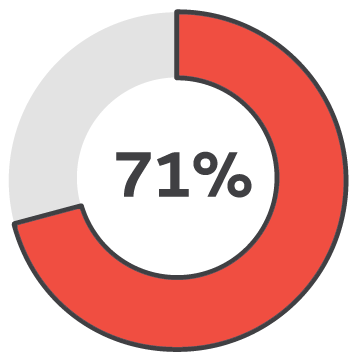
Early intervention with PICO sNPWT resulted in 94% of wounds being healed or a healing trajectory, demonstrated in wounds that were less than 3 months old. In contrast, the success rate dropped to 33% for wounds treated after 12 months.2
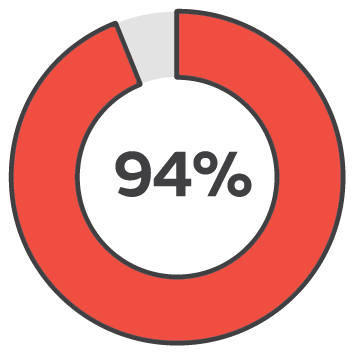
Less than 3 months
Healing success rate starting PICO sNPWT within 3 months.2
3-6 months
Healing success rate starting PICO sNPWT at 3-6 months.2
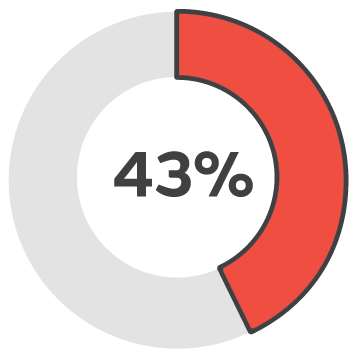
6-12 months
Healing success rate starting PICO sNPWT at 6-12 months.2
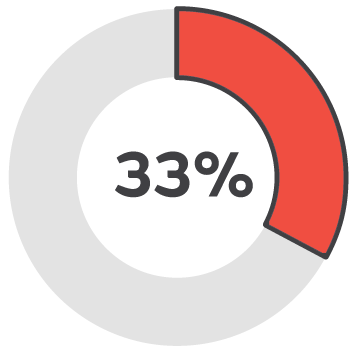
Over 12 months
Healing success rate starting PICO sNPWT over 12 months..2
Understand the relevant criteria for assessing patients/wounds suitable for PICO sNPWT.
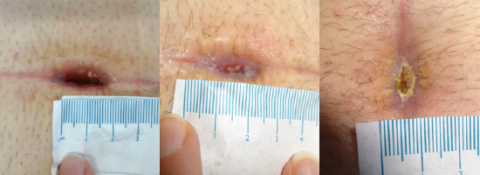
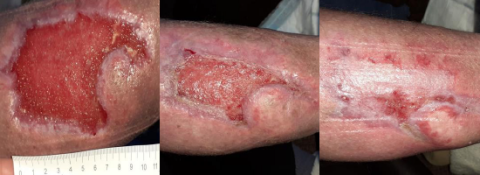
Case studies
PICO System clinical outcomes
How is the PICO System improving lives?
Living with a diabetic foot ulcer can affect so many aspects of a patient’s life. As Kevin’s experience highlights, the PICO System’s ability to offer comfort, freedom and wound healing progress can be truly profound. As you listen to Kevin’s story, imagine the potential benefits for your patients.
Mode of Action references
- Loveluck J, et al. ePlasty. 2016;16:183-195.
- Galiano RD, et al. PlastReconstr Surg Glob Open. 2018;6(1):e1560.
- Pellino G, et al. Surg Innov. 2014;21(2):204–212.
- Kilpadi DV, et al. Wound Repair Regen. 2011;19(5):588-596.
- Malmsjö M, et al. ePlasty. 2014;14:1-15.
- Ma Z, et al. Exp Ther Med. 2016;11(4):1307-1317.
- Xia CY, et al. Mol Med Rep. 2014;9(5):1749-1754.
- Birke-Sorensen H, et al.. Journal of plastic, reconstructive and aesthetic surgery: JPRAS 64 Suppl, S1–S16 (2011)
- Scalise A, et al. Int Wound J. 2016;13:1260–1281.
- Shim HS, et al. Biomed Res Int. 2018;2018
- R. G, R. D, J. S, et al. The effects of a single use canister-free Negative Pressure Wound Therapy (NPWT) System* on the prevention of post surgical wound complications in patients undergoing bilateral breast reduction surgery. Paperpresented at: The British Association of Aesthetic Plastic Surgeons (BAAP's) 30th Annual Scientific Meeting; 2014; London.
- Hudson DA, Adams KG, Van Huyssteen A, Martin R, Huddleston EM. Simplified negative pressure wound therapy: clinical evaluation of an ultraportable, no-canister system. Int Wound J. 2015;12(2):195-201.
- Kirsner R, Dove C, Reyzelman A, Vayser D, Jaimes H. A Prospective, Randomised, Controlled Clinical Trial on the Efficacy of a single-use Negative Pressure Wound Therapy System, compared to Traditional Negative Pressure Wound Therapy in the Treatment of Chronic Ulcers of the Lower Extremities. Wound Repair Regen. 2019;27(5):519 - 529.
- Smith+Nephew 2018.Summary of rountine QA testing on MVP of PICO dressings. 2018. Internal Report. DS/18/153/R.
- Smith+Nephew 2019.Use of Moisture Vapour Permeability* (MVP) and Moisture Vapour Transmission rate** (MVTR) data to support product claims referring to moist wound healing. Internal Report. EO.AWM.PCSgen.001.v2.
- Saxena V, et al. Plast Reconstr Surg. 114:1086–96.
- Borquist O, et al. Annals of Plastic Surgery. 64: 789–93.
- Ichioka S, et al. Wound Rep Regen. 16(3); 460–465.
PICO active incision management references
- Malmsjö M, Huddleston E, Martin R. Biological Effects of a Disposable, Canisterless Negative Pressure Wound Therapy System. ePlasty. 2014;14:1 - 15
- Hudson DA, Adams KG, Van Huyssteen A, Martin R, Huddleston EM. Simplified negative pressure wound therapy: clinical evaluation of an ultraportable, nocanister system. Int Wound J. 2015;12(2):195-201.
- Payne C, Edwards D. Application of the Single Use Negative Pressure Wound Therapy Device (PICO) on a Heterogeneous Group of Surgical and Traumatic Wounds. ePlasty. 2014:152-166.
- Stryja J, Staffa R, Říha D, Stryjová K, Nicielniková K. Cost-effectiveness of negative pressure wound therapy in outpatient setting. Prolekare. 2015;94(8):322 - 328.
- Smith+Nephew November 2018.The Review Of Evidence Supporting The Use Of PICO In Wounds ≥2cm In Depth. Internal Report. EO.AWM.PCS230.001.v2.
- Casey C. Consistent delivery of therapeutic negative pressure levels by a singleuse negative pressure wound therapy system (sNPWT)* in a wound model. Paper presented at: EWMA; 2019; Gothenburg, Sweden
- Smith+Nephew January 2019.Air Leak Tolerance Report: A comparison of PICO v2 (PICO 7 and PICO 14) Devices to PICO vl.6 (PlCO) Devices. Internal Report. RD/19/006.
- Smith+Nephew December 2018.PICO 14 Service Life Testing: 14 Day Device Lifespan. Internal Report. RD/18/132.
- Smith+Nephew 2021.PICO™ Pressure Mapping Study. Internal Report. DS/19/211/R - Part B.
- Smith+Nephew July 2018.PICO 7Y Non-NPWT Wound Model Summary. Internal Report. DS.18.260.R.
- Smith+Nephew 2020.Bacterial barrier testing of the PICO dressing. Internal Report. 2001002.
- Smith+Nephew 2018.Summary of rountine QA testing on MVP of PICO dressings. 2018. Internal Report. DS/18/153/R.
- Gilchrist B, Robinson M, Jaimes H. Performance, safety, and efficacy of a single use negative pressure wound therapy system for surgically closed incision sites and skin grafts: A prospective multi-centre follow-up study. Paper presented at: SAWC; 2020; Virtual
- Sharpe A, Myers D, Searle R. Using single use negative pressure wound therapy for patients with complicated diabetic foot ulcers: an economic perspective. Wounds UK. 2018;14(3):80-137.
- Myers D, Sharpe A. EP549 Service Delivery in Complex DFU Patients using Single Use NPWT - A UK Perspective. Paper presented at: EWMA; 2018; Krakow.
- Hurd T, Gilchrist B. Single use negative pressure wound therapy (sNPWT) in the community management of chronic open wounds deeper than 2cm. Paper presented at: Symposium on Advanced Wound Care/Wound Healing Society Meeting; 2020; Abu Dhabi.
- Kirsner R, Dove C, Reyzelman A, Vayser D, Jaimes H. A Prospective, Randomised, Controlled Clinical Trial on the Efficacy of a single-use Negative Pressure Wound Therapy System, compared to Traditional Negative Pressure Wound Therapy in the Treatment of Chronic Ulcers of the Lower Extremities. Wound Repair Regen. 2019;27(5):519 - 529.
- Wang E, Tang R, Walsh N, et al. Topical negative pressure therapy and compression in the management of venous leg ulcers: a pilot study. Wound Practice and Research 2017;25(1):36-40.
- Smith+Nephew 2020.Negative Pressure Wound Therapy and Pressure Garment Therapy. Internal Report. EO.AWM.PCS261.002.v2.
General references
- Kirsner R,et al Wound Repair Regen. 2019,27(5),519 - 529
- Kirsner RS. et al. Wound Manag & Prev. 2020:66(3):30-36.
- Dowsett C, E’t al. wounds lnternat,onal 2017;8(2):52-58.
- Guest Jf, et al. J Wound Care. 2017;26(6):292-303.
- Fletcher J. et at Wounds UK. 2022. Available at, http://www.pcdsociety.org/resources/details/active-treatment-non-healing-wounds-community
- Nursery research - TSO
- UK survey - citalton TBD
- Smith+Nephew 2018. Internal Report. OS 18.260.R.
- Hurd T. et al. Ostomy Wound Manage 2014;60(3):30-36
- Gilchrist B. et al Performance, safety, and efficacy of a single use negative pressure wound therapy system for surgically closed incision sites and skin grafts: A prospective multi-centre follow-up study. Paper presented at: SWC; 2020.
- Smith+Nephew 2018. Internal Report. RD/18/137.
- Smith+Nephew March 2018. Internal Report, DS.18.066.R.
- Stryja J, et al Prolekare. 2015;94(8):322 - 328.
- Wang E. et al Wound Practice and Research 2017;25(1):36-40.
- Smith+Nephew 2020. lntemal Report. EO.AWM.PC5261.002.v2.
- Smith+Nephew 2018. Internal Report. EO.AWM.PCS230 001.v2.
- Smith+Nephew 2015. Internal Report. ST865 CT09/02.
- Hudson DA., et al. Int Wound J 2015;12(2):195-201
- Payne C., et al. ePlasty. 2014:152-166.
- Casey C., Consistent delivery of therapeutic negative pressure levels by a single use negative pressure wound therapy system (sNPWT)• In a wound model. Paper presented at: EWMA; 2019; Gothenburg, Sweden.
- Smith+Nephew 2019.P ICO Biomechanical Study. Internal Report. DS/19/211/R.
- Malmsjö. et al. ePlasty. 2014;14: l-15.
- Smith+Nephew 2020. Internal Report, 2001002
- Kilpadi DV, et al. Wound Repair Regen. 2011;19(5):588-596.
- Ma Z., et al E,p Ther Med 2016;11(4):1307-1317
- Xia CV., et al Mot Med Rep. 2014;9(5):1749-1754.
- Brownhill VR., et al. Adv Wound Care (New Rochelle) 2020;0(0): l-12.
- Patel A., et al. Comparison of wound closure in chronic lower extremity ulcers between single use negative pressure wound therapy and traditional negative pressure wound therapy: a real world analysis. Paper presented at: National Wound Conference; 2019: Las Vegas, NV, USA
- Hurd T., Gilchrist B., Single use negative pressure wound therapy (sNPWT) in the community management of chronic open wounds deeper than 2cm. Paper presented at: Symposium on Advanced Wound Care/Wound Healing Society Meeting: 2020; Abu Dhabi
- Mcmanus H., et al Bacterial retention within a multi-layered absorbent AIRLOCK’” Technology Single Use Negative Pressure Wound Therapy (sNPWT) dressing. Paper presented at EWMA: 2018; Krakow, Poland,
- Smith+Nephew. 2018. Internal Report. C5DAWM.24.056.
- Smith+Nephew. Internal Report. RD/19/006
- Smith+Nephew. Internal Report RD/18/132.
- Smith+Nephew 2018. Internal Report. DS/18/219/R V2
- Schwartz JA, et al. J Wound care. 2015;24(2).
- Smith+Nephew 2020. Internal Report, EO.AWM.PCS26l 002.v2
- Smith,Nephew 2016. Internal Report. DS.16.179.R.
- Smith+Nephew 2016. Internal Report DS.16.174.A.
- Smith+Nephew 2008. Internal Report. 05/08/062/Rl
- Smith+Nephew 2009. Internal Report DS/08/078/R2
- Smith+Nephew 2008. Internal Report. 05/08/062/R2
- Aicher B, et al. Journal of Vascular Nursing. 2017 Sep 1;35(3):146–56.